What Are the Expected Bond Angles in Icl4+
What are the expected bond angles inICL4. Therefore the bond angle of SiH 4 is 1095.

What Are The Expected Bond Angles In Ici4 Quora
Choose all that apply.
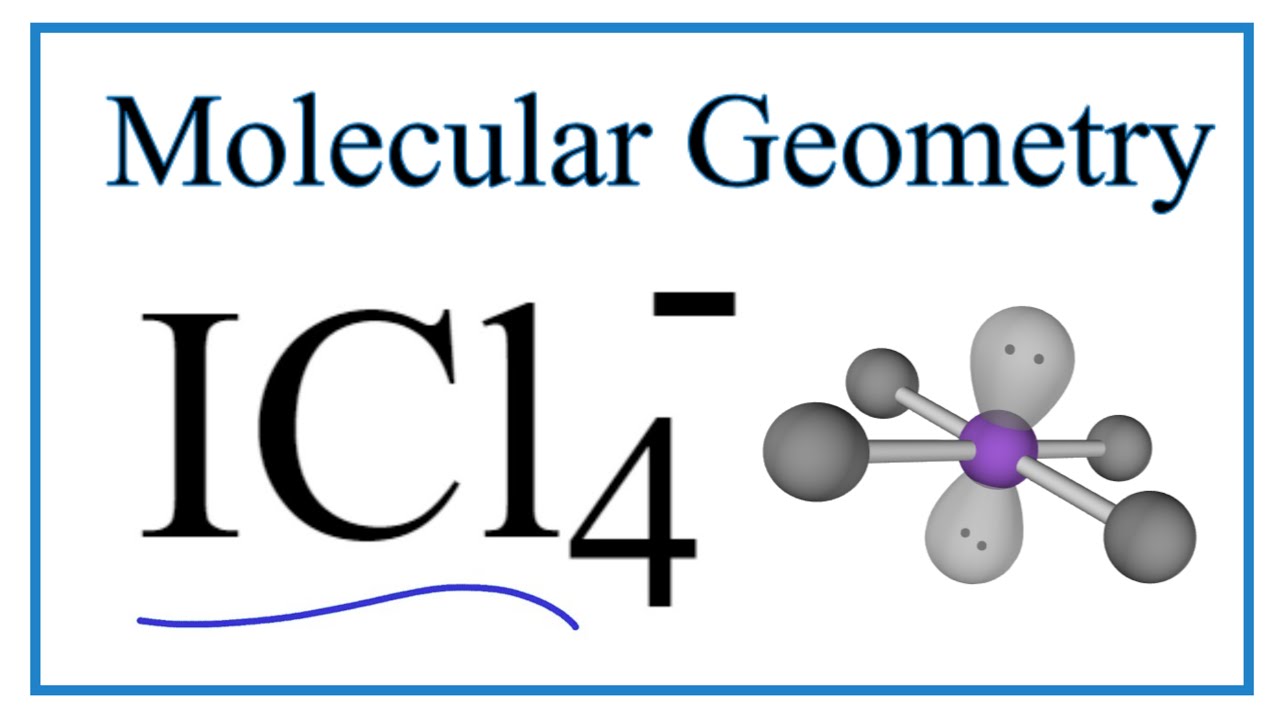
. Based on this information determine the IPI bond angle the ClPCl bond angle and the IPCl bond angle. Therefore the bond angles in ICl 4 are 90 120 and 180. A three-year bond has 80 coupon rate and face value of 1000.
What are the expected bond angles of ICl4. Base of 38 304 plate. Experts are tested by Chegg as specialists in their subject area.
Finally Which has the smallest bond angle Thus OsF2 has the smallest bond angle of the F-S-F bond. However when the electrons surrounding the central atom are lone pairs no bond pairs then the shape of. The bond angle is 120 o where all the atoms are in one plane.
Since there are 4 electron groups around carbon the electron geometry is tetrahedral whose ideal bond angle is 1095. Check all that apply. Carbon is surrounded by 4 electron groups.
A 90 degrees b1095 degrees c120 degrees d180 degrees We have enabled User registrations. Assign hybridization at each atom of the given caffeine molecule. What is the value of the bond angles in sih4 SiH 4.
Up to 256 cash back ICl4- 2. Bond angles and polarity for trans-PF3Cl2. What are the expected bond angles of ICl4.
When you draw the lewis structure of ICl4 you get bond angles of 90120and 180 degrees. Therefore the bond angle of SiH 4 is 1095. Carbon is surrounded by 4 electron groups.
The lone pair would have a 180-deg angle with one chlorine and ninety degree angles with the three equatorial chlorines. If the yield to maturity on the bond is 10 calculate the price of the bond assuming that the bond makes semi-annual coupon interest payments. Answer 1 of 3.
Therefore the bond angle of SiH 4 is 1095. Select all that apply. Resonance structures - chemistry.
What is the bond angle of ICl4. NH2- NH3 and NH4 have H-N-H bond angles of 105 107 and 109. BF3 BeF2 CF4 NF3 OF2.
The central atom bonds with each of the surrounding atoms which form bond angles of 1095 degrees. A 90 degrees b 1095 degrees c 120 degrees d 180 degrees. Which statement best describes the polarity of CF2Br2.
4 bonds and 0 lone pairs. What are the expected bond angles in icl4. View Available Hints 90 degrees 1095 degrees 120 degrees 180 degrees.
Arrange the following AFn species in order of increasing F-A-F bond angles. The Lewis structure of CCl 4 is. The Lewis structure of CCl 4 is.
In general stable structures need to have lone pairs as far away from other electrons as possible. 90 degrees 120 degrees 180 degrees. What is the value of the bond angles in ccl4ccl4.
Which molecule in each pair is more polar. The third function is operated via a toggle in the lever handle. Choose all that apply.
Choose all that apply. Part B What are the expected bond angles in ICLA. What is the value of the bond angles in ccl4ccl4.
Find step-by-step Chemistry solutions and your answer to the following textbook question. 90 degrees 1095 degrees 180 degrees 120 degrees. Is CO trigonal planar.
ICl4- Yes COF2 Yes TeCl4 no XeF2 no. Use VSEPR theory to predict the bond angles of sulfur hexafluoride. Arrange the following molecules in order of increasing bond angles.
1 ClO2 or SO2 2 HBr. What are the expected bond angles of ICl4. 4 bonds and 0 lone pairs.
What are the expected bond angles of ICl4. Do ICl4- COF2 TeCl4 XeF2 have possible resonance structures. Three ninety degree angles are not good.
Since there are 4 electron groups around silicon the electron geometry is tetrahedral whose ideal bond angle is 1095. Explain this variation in bond angles. We see from Figure 92.
What are the expected bond angles in ICl4. What are the expected bond angles in ICl4. If it is non polar the bond angles are as followsI-P-I bond angles.
3 that the molecular geometry of CO 3 2 is trigonal planar with bond angles of 120. According to vsepr theory when the electrons surrounding the central atom are bond pairs the shape of the molecule will depend upon the type of Hybridization. Choose all that apply.
Who are the experts. What are the bond angles of PI3Br2. What I am thinking.
Check all that applies. Silicon is surrounded by 4 electron groups. A 90 degrees b1095 degrees c120 degrees d180 degrees.
SF6 IF5 XeF4 SeO3. A 90 degrees b1095 degrees c120 degrees d180 degrees. Since there are 4 electron groups around carbon the electron geometry is tetrahedral whose ideal bond angle is 1095.
What is CO molecular structure. What are the expected bond angles in ICl 4. A Film by CommonMan the sequel to I Called The Law 3.
A tetrahedral has a central atom surrounded by four other atoms.
What Are The Expected Bond Angles In Ici4 Quora
What Are The Expected Bond Angles In Icl4 How To Discuss

Icl4 Molecular Geometry Bond Angles Electron Geometry Youtube
0 Response to "What Are the Expected Bond Angles in Icl4+"
Post a Comment